New Agent For Detecting Location of Recurring Prostate Cancer
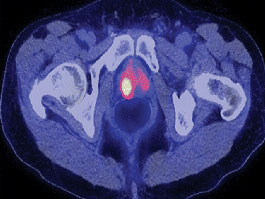
The FDA approved the production and use of Choline C 11 injections to help detect recurrent prostate cancer.
This PET imaging agent is especially useful when PSA levels are increasing after earlier treatment for prostate cancer but conventional imaging tests don’t show signs of cancer.
The Choline C 11 injection is administered intravenously to produce an image that helps to locate specific body sites for follow-up tissue sampling and testing in men with recurrent prostate cancer.
It does not replace tissue sampling and testing but assists in identifying areas for sampling when PSA increases indicate a recurrence but other results have not shown cancer.
The Mayo Clinic is the first FDA-approved facility to produce Choline C 11 injection. Choline C 11 injection must be produced in a specialized facility and administered to patients shortly after its production.
In studies submitted to the FDA, at least half of the patients who had cancers detected on PET scans with Choline C 11 also had recurrent prostate cancer confirmed by tissue sampling of the same area.
False positives were also reported in 15 to 47 percent of patients, underscoring the need to confirm results with tissue samples.
Identifying the location of recurrent prostate cancer is important for further treatment decisions.











