Prostate Specific Antigen (PSA) Test Procedure Standardization Dilemma
In recent years, it has become evident that the same patient, who has more than one PSA test, can have different PSA results if the blood samples were sent to different laboratories.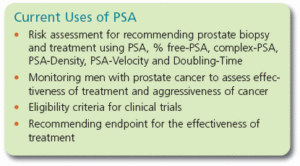
Now, we know these differences in reported values are due primarily to differences in the standardization of different PSA tests.
Also, it has become increasingly clear that potential differences in clinical interpretation of PSA results have not been fully appreciated by the medical community.
Nor has the topic been presented adequately to the millions of men being screened annually for prostate cancer with the PSA test.
Two Standards for the PSA Screening Test for Prostate Cancer
Today, two different standards – Hybritech* and WHO (World Health Organization) – exist for measuring PSA levels, and these standards do not produce equivalent results.
In a study, in which a screening population of 2,300 men was tested using the Hybritech and WHO standards, the WHO tests yielded PSA levels approximately 23% lower than the Hybritech ones.
The Hybritech PSA is considered to be the true PSA because virtually all the cutoffs currently used in clinical practice for biopsy were established using the Hybritech test and standard.
The WHO didn’t know there would be a discrepancy between the results. They thought they were providing a gold standard that would be used all over the world.
It would be fine for every manufacturer to standardize to the WHO standard now, but they would have to change all of the PSA parameters that have been established using the Hybritech standard, which nobody wants to do.
Consequences on PSA Test Accuracy
The problem is that lower PSA results with WHO standardization can lead to different diagnostic and clinical outcomes.
The PSA value in itself is important to the early detection and treatment of prostate cancer, but physicians also use other information derived from the PSA test to make more informed decisions about the risk of cancer and the need for biopsy: PSA-Velocity, PSA-Doubling Time, PSA-Density and free-PSA.
For all these measurements, accuracy and consistency of PSA results are critical.
But it’s not just early detection that is affected by these different standards.
Every aspect of PSA diagnostics, including follow-up tests after treatment, ultimately relies on the standardization of the individual test results relative to previously established clinical decision points.
Numbers Affected
15 million US men are screened for prostate cancer each year with PSA tests. 7.5 million of those PSA results come from kits aligned to Hybritech standard material and 7.5 million are from kits aligned to WHO (World Health Organization standard material.
Based on a community screening in Houston of 2,304 men, 2% had PSA levels below 4.0 ng/mL using WHO-aligned kits, but above 4.0 ng/mL on Hytybritech-aligned kits.
Applying that math to national figures, 150,000 men would be affected by this different testing standard. And we know that 27% of men in the 3 to 4 ng/mL PSA range are expected to have prostate cancer found on biopsy, approximately 40,500 US men each year from those screened.
Whether using the 2.5 ng/mL or the 4.0 ng/mL cutoff for recommending biopsy, the Hybritech-standardized test detects approximately 5% more men who would be recommended for biopsy compared to the WHO standardized test.
Taking Two PSA Tests with Different Standards
The following are examples to show the possible consequences of taking two PSA tests and not knowing they are going to be measured with two different standards.
In the first situation, the patient takes a PSA test, which uses a Hybritech standard, and gets back a 4.1 ng/mL value. He takes a follow-up test, which uses a WHO standard, and gets back a 3.2 ng/mL value – reflecting the 23% difference resulting from the WHO standard.
Because of the lower PSA in the second test, the following occurs: If the 4.0 PSA cut off is being used for a biopsy recommendation, a delay in a prostate biopsy will occur.
Because the second test has a lower value, it will look as if the PSA-Doubling Time is longer, the PSA-Velocity is lower and the PSA-Density is lower than each actually is.
In a second situation, the patient takes a PSA test, which uses the WHO standard, and gets backs a PSA reading of 4.1. He takes a follow-up test, which uses a Hybritech standard, and gets back a PSA 5.0.
Because of the lower PSA in the first test and the higher PSA in the second, the following has occurred:
A biopsy was recommended for the 4.1 but it should have been recommended earlier because it’s now happening when the PSA is actually 5.0.
The PSA- Density appears lower than it actually is. In this example, the PSA-Density for a 30cc gland should be 5.0 divided by 3 rather than 4.1 divided by 3.
The PSA-Doubling Time is shorter because going from WHO to Hybritech would double a value of 4.1 (to 8.2) more quickly.
The PSA-Velocity would appear higher because even if the PSA remained the same if the same standard had been used, now it appears that it went from 4.1 to 5.0.
No Excuses
A few years ago, it would be possible to explain that accuracy of prostate cancer assessment through PSA screening was compromised due to a largely unrecognized difference in the PSA concentration in common PSA standard materials.
We don’t have that excuse now. We know what is happening.
Recommendations for PSA Test Method
Physicians and patients need to be aware of which standard is being used to measure their PSA so that their results can be interpreted correctly for biopsy recommendation, to assess the aggressiveness of the tumor, to select the patient for inclusion in a clinical trial, and to interpret the response to therapy.
Manufacturers and clinical laboratories should provide the standardization method used for the test results and they should include the result that would be obtained with the alternate PSA standard. Both values should be provided for every test to make comparisons possible.
(This article is an updated version of a previous QUEST article, PSA Tests Are Not All the Same. This information, adapted for QUEST readers, is based on a journal article published in the June, 2007 issue Journal of Urology and Dr. Catalona’s presentation for a prostate cancer meeting in Texas.
*Dr. Catalona led the PSA studies, with research support from Hybritech, that resulted in FDA approval of Hybritech’s PSA assay as an aid to the early detection of prostate cancer. Dr. Catalona is a consultant and investigator for Beckman Coulter Incorporated, a company that purchased Hybritech, and Dr. Catalona receives research support and honoria for consultation and speaking for Beckman Coulter Incorporated. Dr. Catalona has no ownership or investment interest in Beckman Coulter Incorporated.