Determining a PSA Level That Will Define a “No Evidence of Disease”
This article is a follow-up to the article in the Winter 2010 QUEST which introduced the research on Nanotechnology to help make better treatment decisions after a radical prostatectomy. The Nanotechnology PSA test described in this material is not commercially available at the present. We include the information to keep readers informed about the latest research in which Dr. Catalona, his research collaborators and the URF are involved and to show the potential impact of this research and of technological advances in the diagnosis, treatment and prevention of prostate cancer.

 Using nanotechnology to find trace elements of PSA is a powerful tool, but it doesn’t establish the PSA level that gives physicians the information they need to interpret results for recommending additional treatment or not.
Using nanotechnology to find trace elements of PSA is a powerful tool, but it doesn’t establish the PSA level that gives physicians the information they need to interpret results for recommending additional treatment or not.
Using Dr. Catalona’s surgical patients, the Urological Research Foundation supported a study to research what PSA level – using nanotechnology – will inform physicians and patients if prostate cancer is present or not after a radical prostatectomy and what treatments, if any, might be recommended accordingly.
PSA is used to monitor the effectiveness of therapy and recurrence in patients who have undergone treatment for prostate cancer.
Using nanotechnology, this study analyzed banked serum samples obtained from men after radical prostatectomy at a time when all PSA values were undetectable by conventional PSA testing; yet, some men had cancer recurrence.
The study also measured the nanotechnology PSA in men who had received salvage radiation.
Since PSA is the product of prostatic epithelial cells, the complete removal of the prostate after radical prostatectomy, in theory, should be “undetectable” using conventional PSA tests with clinical limits of detection up to 0.1 ng/mL.
By using tests that measure PSA at levels below this clinical limit of detection, the opportunity exists to define a much more sensitive PSA level in men who have undergone radical prostatectomy.
The nanotechnology PSA test developed by researchers at Northwestern (Shad Thaxton PhD, MD and Chad Merkin PhD) is able to measure approximately 300 times more sensitive than current commercial PSA tests.
But what do the results mean and how should they be used?
The objective of the URF study using Dr. Catalona’s patients’ blood samples was to use nanotechnology to measure PSA after radical prostatectomy and determine a PSA level that will define a “no evidence of disease” status.
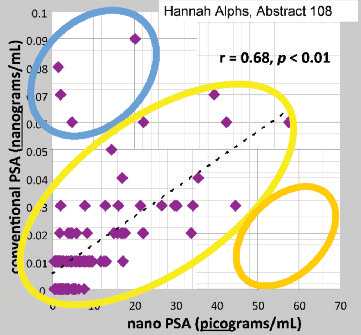
The study found that men with long-term follow-up and no evidence of cancer recurrence had nano-PSA levels that were on average 0.0035 ng/mL compared to 0.0286 ng/mL (almost 9 times higher) in men who had a recurrence of their prostate cancer.
The study proposes a nPSA of 0.0175 ng/mL (nanograms per mililiter) which is the same as 17.5 pg/mL (picograms per milliliter) – the 90th percentile – as a cut-off for disease-free status after radical prostatectomy. (A minute amount of PSA is produced by the remaining urethral glands.)
To get away from all of the zeros, the finding is expressed in picograms rather than nanograms. (A picogram = 1/1000 of a nanogram.)
Comparing patients whose tumor was completely confined to the prostate gland and therefore were at low-risk for recurrence with other patients who had been successfully treated with salvage radiation therapy, the nano-PSA levels were lowest (1.5 picograms/mL) in the salvage radiation patients because the salvage radiation also destroyed the PSA-producing cells in the urethra.
(based upon study and AUA presentation: What is a Normal Nanotechnology PSA Following Radical Prostatectomy? Lee C. Zhao; Dae Y. Kim; Danie’ C. O’Brien; Donghui Kan; Norm D. Smith, MD; Stacy Loeb, MD; Chad A. Mirkin, PhD; C. Shad Thaxton, MD; William J. Catalona, MD)







