Two PSA Test Standards Are Causing Problems in Screening for Prostate Cancer
Two different standards – *Hybritech and WHO (World Health Organization) – exist for measuring PSA levels, and these standards do not produce equivalent results.
Having two standards means the same blood sample sent to labs using different test calibrations would come back with two different PSA results.
Studies show that WHO tests yield PSA levels approximately 20-23% lower than the Hybritech ones.
Problems Created
The effects of this difference result in the following:
- Some men who should be referred for biopsy aren’t.
- Important diagnostic derivatives of PSA – PSA Velocity, PSA Density, and PSA Doubling Time – are impacted by the different test results.
- The differences affect treatment evaluations for prostate cancer patients and even acceptance into clinical studies.
- Patients managed with “watchful waiting,” using PSA tests with inconsistent standards aren’t able to identify disease progression.
PSA Is Important Test
PSA is the most important test for early detection of prostate cancer and for treatment evaluation. New markers for prostate cancer will complement and maybe even replace PSA at some point, but not now.
Now, patients and doctors depend on PSA. And they need to understand and interpret the effects of these two different testing standards.
Virtually all the cutoffs currently used in clinical practice for biopsy recommendations were established using the Hybritech test and standard.
The WHO didn’t know there would be a discrepancy between the results of the two tests. They thought they were providing a gold standard that would be used all over the world.
But to standardize to the WHO test: 1. Manufacturers would have to change all of the PSA parameters established using the Hybritech standard and 2. Research studies based upon these standards would all have to be re-evaluated.
The tasks are unrealistic and potentially mistake-ridden. It appears that both manufacturing standards are here to stay.
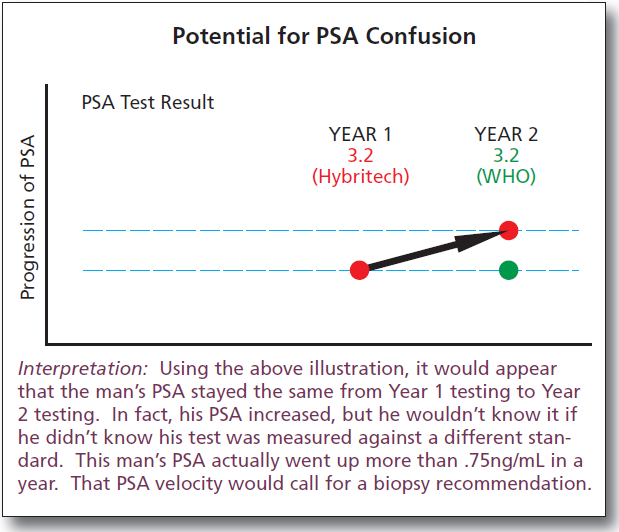
The following are examples to show the possible consequences of taking two PSA tests and not knowing they are going to be measured with two different standards.
First Situation
The patient takes a PSA test, which uses a Hybritech standard, and gets back a 4.1 ng/mL value. He takes a follow-up test, which uses a WHO standard, and gets back a 3.2 ng/mL value – reflecting the 23% difference resulting from the WHO standard.
Because of the lower PSA in the second test, the following occurs: If the 4.0 PSA cut off is being used for a biopsy recommendation, a delay in a prostate biopsy will occur.
Because the second test has a lower value, it will look as if the PSA-doubling time is longer, the PSA-velocity is lower and the PSA-density is lower than each actually is.
Second Situation
The patient takes a PSA test, which uses the WHO standard, and gets backs a PSA reading of 4.1. He takes a follow-up test, which uses a Hybritech standard, and gets back a PSA 5.0.
Because of the lower PSA in the first test and the higher PSA in the second, the following has occurred:
A biopsy was recommended for the 4.1 but it should have been recommended earlier because it’s now happening when the PSA is actually 5.0.
The PSA-density appears lower than it actually is. In this example, the PSA-density for a 30cc gland should be 5.0 divided by 3 rather than 4.1 divided by 3.
The PSA-doubling time is shorter because going from WHO to Hybritech would double a value of 4.1 (to 8.2) more quickly.
The PSA-velocity would appear higher because even if the PSA remained the same if the same standard had been used, now it appears that it went from 4.1 to 5.0.
What To Do
The solution for eliminating the problems and the confusions is patient and doctor awareness, knowledge and attention to detail.
Important clinical decisions would be made erroneously using WHO tests unless doctors were aware of its different scoring from Hybritech tests.
Physicians and laboratories need to understand what’s happening and how to convey risks for prostate cancer from PSA test results to patients.
Doctors should ask labs which PSA test is used and then know the appropriate PSA biopsy-recommending threshold for that test.
Past PSA tests – including PSA derivatives – should be analyzed, especially for men who have PSA 2.0 ng/mL or higher values to make sure results are accurate and consistent diagnostic measurements.
African American and high-risk men need to be retested and their PSA scores reinterpreted if it’s not known whether and when the Hybritech and WHO standards were used.
Physicians and patients need to be aware of which standard is being used to measure PSA so that results can be interpreted correctly for biopsy recommendations, to assess the aggressiveness of tumors, to select patients for inclusion in clinical trials, and to interpret responses to therapy.
Manufacturers and clinical laboratories should provide the standardization method used for the test results and they should include the result that would be obtained with the alternate PSA standard. Both values should be provided for every test to make comparisons possible.
Every year, more than 20 million men take a PSA test for prostate cancer screening.
More than 10 million of those tests are calibrated using one standard and more than 10 million using another.
The WHO standard results come back 20-23% lower than the Hybritech standard. (The Hybritech standard has been the clinical standard used for biopsy recommendations since 1994, the beginning of PSA screening.)
Of those more than 20 million, statistically, 1 man in 20 (1 million) will have a PSA from 3 to 4 ng/ml. But it doesn’t work that way because half of that 20 million tested with the WHO standard are getting lower results by at least 20%.
Five hundred thousand men each year are getting PSA results that should indicate a need for biopsy – according to well tested clinical studies – but their WHO test results mislead them into thinking they’re in the “safe” group.
The difference would be important if even one man’s cancer was missed but the numbers make it a serious problem.
Approximately 25% of men with PSA results from 3 to 4 will have prostate cancer and 1/4 of that group will have aggressive cancers.
So, statistically, 25% of those five hundred thousand men who took the WHO test (125,000) will have prostate cancer and at least a quarter of those men (31,000) will have aggressive cancers.
The problem is that if a PSA 4 cutoff is used for a biopsy recommendation (and now many doctors recommend even lower at 2.5), those men who got their results from the WHO standard will get back results that don’t sound the alarm. Biopsies won’t be recommended.
For example, if a man’s blood sample was sent to two laboratories with two different standards and it came back 4 from the Hybritech standard, it would come back 3.2 from the WHO standard. The Hybritech report would most likely result in a recommendation for biopsy; whereas, the WHO report would not (if PSA 4 was being used for the biopsy threshold).
Prostate cancers could be found one to two years later in the men who got their results from the WHO test than those from the Hybritech test.
*Dr. Catalona led the initial PSA studies, with research support from Hybritech, that result in FDA approval of Hybritech’s PSA assay as an aid to the early detection of prostate cancer. Dr. Catalona is a consultant and investigator for Beckman Coulter, a company that purchased Hybritech. Dr. Catalona receives research support and honoraria for consultation and speaking for Beckman Coulter. Dr. Catalona has no ownership or investment interest in Beckman Coulter Incorporated.









